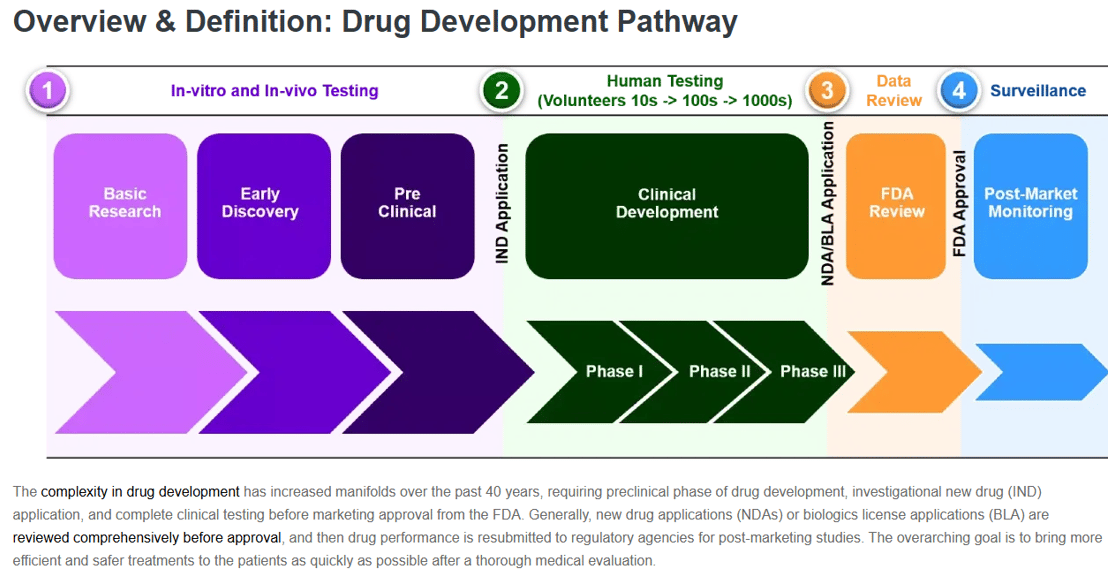
Drug Development
The drug development process typically involves five main stages: discovery and development, preclinical research, clinical research, FDA review, and post-market safety monitoring. This process is a multi-year endeavor, costing billions of dollars and requiring extensive research and testing to ensure safety and efficacy.
Elaboration:
1. Discovery and Development:This initial stage involves identifying potential drug targets, often a specific molecule or gene, and then designing or selecting molecules that may interact with the target to alter disease.
2. Preclinical Research:This stage involves testing the potential drug in laboratory settings, including computer models and cell cultures, and in animals to evaluate its effects and safety.
3. Clinical Research:This phase involves testing the drug in human subjects through clinical trials, which are typically divided into four phases: Phase I (safety), Phase II (efficacy and dosage), Phase III (confirmatory efficacy and safety), and Phase IV (long-term safety and efficacy).
4. FDA Review:Once the drug has successfully completed clinical trials, the pharmaceutical company submits an application for approval (e.g., NDA) to the FDA, which reviews the data and makes a decision on whether to approve the drug for use in humans.
5. Post-Market Safety Monitoring:Even after a drug is approved, it undergoes ongoing monitoring to track its long-term safety and efficacy and to identify any potential side effects or issues that may have not been detected during clinical trials.
(From Northeast Biolab)
Device Develop Process
Device development encompasses the entire lifecycle of bringing a new device to market, from initial concept and research to manufacturing, testing, and regulatory approval. It's a complex process involving various stages and considerations, particularly for medical devices where safety and efficacy are paramount.
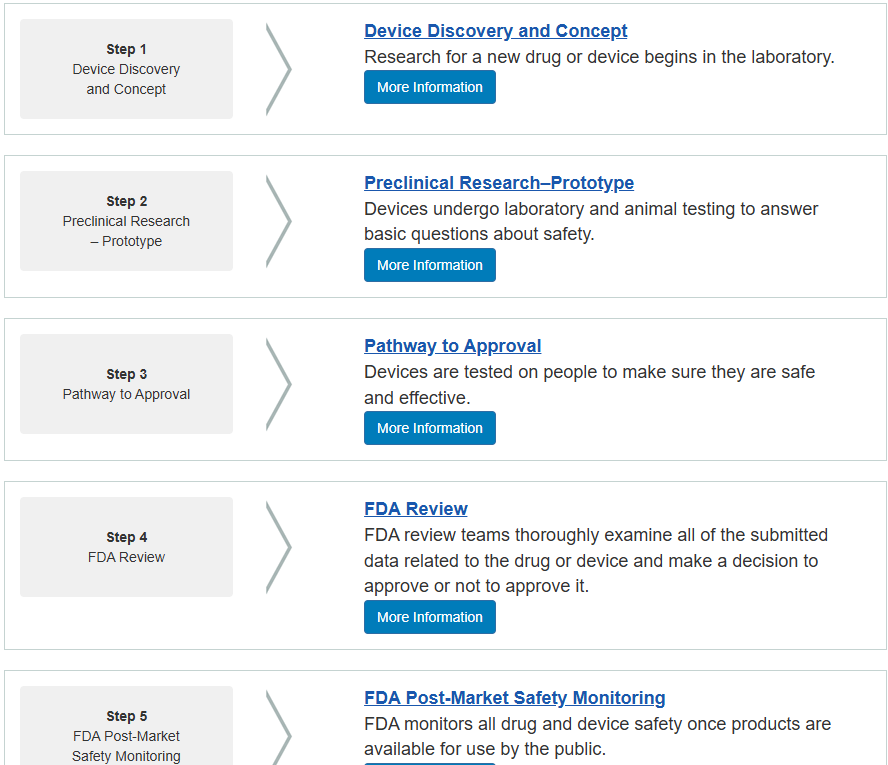
Medical Device Development Specifics:
Medical devices are classified based on risk levels (Class I, II, and III), which determine the level of regulatory scrutiny and testing required.
The FDA and other regulatory bodies have specific requirements for medical device development, including clinical trials, quality management systems, and post-market surveillance.
Medical device development often involves clinical trials to evaluate the device's safety and effectiveness in humans.
Even after a device is approved and launched, ongoing monitoring and reporting are required to ensure its safety and effectiveness.
In summary, device development is a multi-stage process that requires careful planning, execution, and adherence to regulatory requirements. It is a collaborative effort involving engineers, scientists, and regulatory experts, with the ultimate goal of delivering a safe, effective, and reliable product to the market.
In Vitro Diagnostics Development
The in vitro diagnostics (IVD) product development process typically involves several key stages, from initial concept to market launch, including feasibility assessment, design and development, validation, regulatory approval, and manufacturing/scale-up. This process is often guided by a phase-gate model, with each phase requiring specific activities and approvals.
Here's a more detailed breakdown
1. Concept and Feasibility:
Idea Generation: Identify unmet clinical needs and potential solutions.
Market Research: Analyze existing IVD products and identify opportunities.
Feasibility Studies: Evaluate the technical and economic viability of the proposed IVD.
Target Product Profile (TPP): Define the specific characteristics and performance requirements of the IVD.
2. Design and Development:
Product Design: Develop the design for manufacturing (DFM), including assay design, instrumentation, and automation.
Prototype Development: Build and test prototypes to assess performance and functionality.
Analytical Performance Studies: Evaluate analytical sensitivity, specificity, and linearity.
Stability Studies: Determine the shelf-life and stability of reagents and test strips.
Clinical Performance Studies: Assess the accuracy and reliability of the IVD in real-world clinical settings.
3. Validation and Approval:
Validation Studies: Confirm that the IVD meets the required performance specifications and regulatory requirements. [3, 3, 8, 20]
Regulatory Submission: Prepare and submit regulatory applications to obtain approval from agencies like the FDA or other relevant authorities.
Premarket Approval (PMA) or other Regulatory Pathways: Navigate the regulatory approval process, which may involve clinical trials, product descriptions, and manufacturing information.
4. Manufacturing and Scale-Up:
Manufacturing Development: Optimize the manufacturing process to ensure cost-effectiveness, quality, and scalability.
Technology Transfer: Transfer the developed assay or instrument to a production facility.
Production and Quality Control: Implement quality control measures to maintain product quality throughout the manufacturing process.
Scale-Up: Increase production capacity to meet market demand.
5. Commercialization and Launch:
Marketing and Sales: Develop a strategy to launch the IVD product and gain market share.
Distribution and Logistics: Establish a distribution network to ensure timely and efficient delivery to customers.
Post-Market Surveillance: Monitor the performance of the IVD in real-world use and address any issues that may arise.
Key Considerations:
Regulatory Compliance: IVDs are subject to stringent regulatory requirements, and manufacturers must comply with all applicable regulations.
Quality Management Systems: Implement a robust quality management system (QMS) to ensure product quality and safety.
Project Management: Effective project management is crucial for coordinating resources, managing risks, and achieving timely product development.
Outsourcing: Companies may choose to outsource certain aspects of the IVD development process, such as manufacturing or clinical trials.
Product Lifecycle Management (PLM): Manage the IVD product throughout its lifecycle, from design and development to launch, maintenance, and end-of-life.
